You are about to be redirected to Xellia’s global website
We are now redirecting you to our global website, click ‘confirm’ below to continue or ‘cancel’ and we will return you to your previous page.
Cancel ConfirmClick here to see full
Prescribing Information
including BOXED WARNING.
Unique use of excipients in
VANCO READY® formulation
VANCO READY® has a BOXED WARNING
with absolute contraindication in
pregnant women.
By utilizing Safe Implementation Strategies and the additional safety protocols of systems such as EPIC, Meditech, and Cerner.
“We have been running 6 months with no issues.”
- Pharmacy Director
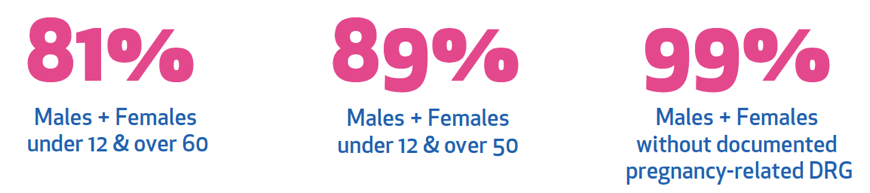
"We give it to men, women outside childbearing ages and women of childbearing age post-negative pregnancy test.”
- Pharmacy Director
VANCO READY® may help reduce the potential risk for errors associated with compounding drugs.
Learn MoreGet the full line of
VANCO READY®. 7 different doses from 500 mg to 2 g.
VANCO READY® Vancomycin Injection, USP is available in 7 different dose amounts to cover most of your patients' vancomycin needs.
Order VANCO READY® NOW
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use
VANCOMYCIN INJECTION, safely and effectively. See full prescribing
information for VANCOMYCIN INJECTION.
VANCOMYCIN injection, for intravenous use
Initial U.S. Approval: 1958
RECENT MAJOR CHANGES
Boxed Warning 10/2021
Warnings and Precautions, Severe Dermatologic Reactions (5.5) 5/2021
Warnings and Precautions, Potential Risk of Exposure to Excipients During the First or Second Trimester of Pregnancy (5.1) 10/2021
WARNING: POTENTIAL RISK OF EXPOSURE TO EXCIPIENTS DURING THE FIRST OR SECOND TRIMESTER OF PREGNANCY
See full prescribing information for complete boxed warning.
If use of vancomycin is needed during the first or second trimester of pregnancy, use other available formulations of vancomycin. This formulation of vancomycin injection contains the excipients polyethylene glycol (PEG 400) and N-acetyl D-alanine (NADA), which resulted in fetal malformations in animal reproduction studies at dose exposures approximately 8 and 32 times, respectively, higher than the exposures at the human equivalent dose (5.1, 8.1).
To reduce the development of drug-resistant bacteria and maintain the effectiveness of Vancomycin Injection and other antibacterial drugs, Vancomycin Injection should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. (1.6)
DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
Vancomycin Injection, USP: Single-dose flexible bags containing 500 mg vancomycin in 100 mL, 750 mg vancomycin in 150 mL, 1 g vancomycin in 200 mL, 1.25 g vancomycin in 250 mL, 1.5 g vancomycin in 300 mL, 1.75 g vancomycin in 350 mL and 2 g vancomycin in 400 mL of liquid. (3)
Hypersensitivity to vancomycin (4)
WARNINGS AND PRECAUTIONSADVERSE REACTIONS
The common adverse reactions are anaphylaxis, “red man syndrome”, acute kidney injury, hearing loss, neutropenia. (6.1)